Benefits and risks of using CBD
We are ENFRIARTE, and I tweet random things about ADHD on Twitter with @GixTanv and sell CBN liquid on Mercari and e-commerce sites.
This article aims at providing the information helping understanding the benefits and risks associated with CBD use.
About CBD
Cannabis sativa(marijuana) plant has been used for treatment of many diseases and disorders for long history. As the second most prevalent bioactive constituent of Cannabis sativa, CBD also has the potential. In high-dose use, the expected neurotherapeutic effects include anti-inflammatory, antiepileptic, antianxiety (anxiolytic), antipsychotic. And in low-dose use, the effects of helping solving self-perceived anxiety and sleep issue are wildly reported. Different from Δ9-THC, CBD avoids the unwanted psychoactive side effect and therefore is currently attracting much interests. However, it doesn’t mean that CBD is risk-free.
Two types of CBD use: divided based on the dosage
Based on dosage, the use of CBD can be divided into two categories:
low-dose CBD(~100mg/day [1]): for mitigating problems including self-perceived anxiety, insomnia etc.
high-dose CBD(300mg~ 900mg/day [2][3][4][5]): for treatment of seizures, social anxiety disorders(SAD), anxiety and pain
The latter one could be seen in clinical trials aiming at studying the effects of CBD. For example, in the study conducted by Masataka [2] from Kyoto University, the dosage is 300mg per day, continuing for one month. And in another study carried out by Crippa et al. 2011[4], participants took 400 mg CBD in single dose.
While the former one, based on the online survey conducted in 2021[1], more than half of the participants(N = 373) report their daily dose lower than 50mg, where 29.5% is lower than 25mg, and 24.9% is 25-49 mg.
The difference between high-dose CBD and low-dose CBD use is so large, making it hard to talk about risk in low-dose CBD use using data and conclusion obtained from high-dose clinical trial. But still, to provide a clear view about the current situation, risk and benefits of CBD use, we will cover both types of CBD use in this article.
Risks in CBD use
CBD isn't risk-free. The most common adverse events seen in high-dose CBD use are considered as somnolence and fatigue coupled with gastrointestinal disturbances. Rarer but serious events such as elevated liver function have been also observed [7]. Another thing needed to be mentioned in high-dose CBD use is drug interaction.
Currently there is study [6] showing the drug interaction between CBD and other medications may result in decreased drug effects and unwanted side effects, which is planning to be introduced in detail in our future articles.
And much more research is needed in this topic to determine how to manage patients, especially those with refractory seizures on multiple drugs impacting the CYP enzyme system [7].
Considering the drug interaction and possible averse events, talking to your doctor before starting using CBD product is wildly suggested. However, it may not that realistic not only in Japan but also in other countries, for both the difference between high-dose and low-dose CBD, and the quality control issue in non-FDA-approved CBD product.
About quality control
Currently, there is only one CBD drug product approved by FDA, a prescription drug product called Epidiolex to treat seizures associated with Lennox Gastaut syndrome (LGS), Dravet syndrome (DS), or tuberous sclerosis complex (TSC). Thus, all other CBD products, no matter the purchase source, are non-FDA-approved, which may lead to quality control problems.
In 2016, one investigation carried out in US where the investigators purchased 84 non-FDA-approved CBD products from the internet and tested them in a commercial laboratory [8]. The results showed that, the frequency of accurate labeling for CBD vaporization liquids, tinctures, and oils was 12.5%, 25%, and 45%, respectively. Similar results could be found in the investigation carried out in the Netherlands in 2018 [9], where only 50% of assessed CBD products are labeled correctly.
With such a low accuracy, it’s impossible to know the exact dose of CBD a patient is taking if they buy non-FDA-approved products. The potential implications due to the variable dosage of CBD exists, especially when patient takes high-dose CBD.
One example could be found in seizure control, where even switching from brand antiepileptic drugs to generic drugs or vice versa increased the risk of emergency medical services, which implies the risk of using CBD product with variable CBD concentration [10].
Therefore, using a product tested by an independent laboratory is highly recommended when it’s non-FDA-approved.
User-experience-based effects of low-dose CBD use
Compared to clinical trials where high-dose CBD are considered, the number of research on the effects of low-dose CBD is limited. Recently in 2021, a study was conducted by using online surveying of CBD users to understand patterns of use, dose and self-perceived effects[1]. The sample number is 387 and participants come from different country (77.4% from UK) and vary in age and sex. The CBD purchase locations are mainly online CBD shop(legal), health shop and pharmacy, with only 0.5% of participants report they received the products from medical prescriber.
The results showed that a majority of participant found that CBD helped their symptoms and they often used doses below 50 mg. The findings are summarized as follows:
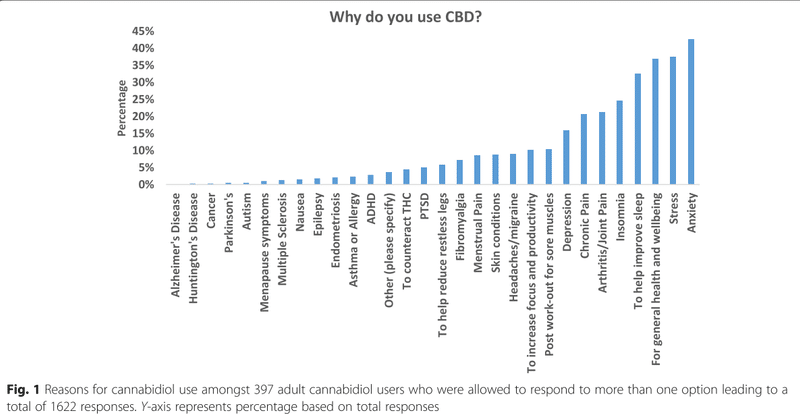
The top reason for using CBD is self-perceived anxiety, reported by 42.6% of participants. Followed by sleep issue, reported by 42.5%. The detail is shown in Fig. 1.
Among the people using CBD for self-perceived anxiety, 86.5% responded that they felt less anxious
Among the 42.5% of participants using CBD for sleep issues, the positive effect of CBD has been reported. The detail is shown in Fig. 2. Specifically, in the questions about the time it takes to fall asleep, 48.2% report that the time needed for falling asleep is shorter, while 17.7% say they didn’t have a problem falling asleep before.
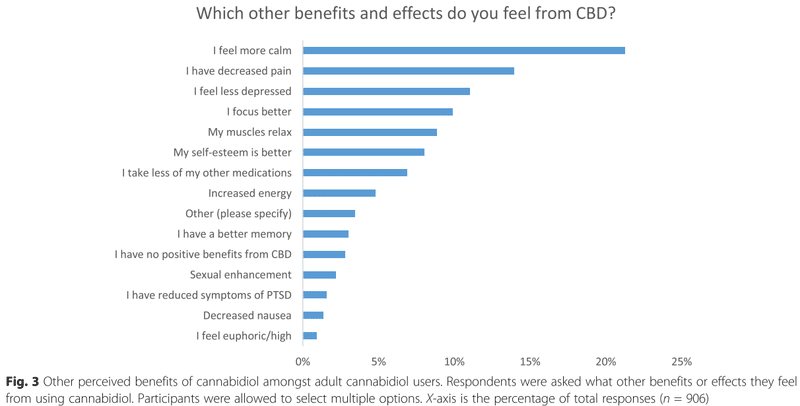
Other benefits are also reported. Among them, the most mentioned is calm, followed by decreased pain. The detail is shown in Fig. 3.

Potential of high-dose CBD use: based on clinical trials
For clinical trials, the major potential of CBD is considered as treatment for seizure disorders, anxiety, schizophrenia, pain.
Among them, CBD working as one solution for seizure disorder is considered effective, which is also reflected in the FDA-approved drug product Epidiolex.
For anxiety, there are many studies assessing the impact of CBD on anxiety and some of them are double-blinded, showed significantly decreased anxiety. One example is the anti-anxiety effects of repeated CBD treatment in teenagers with social anxiety disorders, conducted by Masataka from Kyoto university [2]. Another example could be found in a study related to anxiety before public speaking [5]. These results indicate that CBD is promising option to treat anxiety.
On the topic of CBD working on schizophrenia, there are 3 randomized trials [11][12][13] demonstrating a reduction of schizophrenia symptomatology over time, but the impact of CBD therapy on the disease differs.
When it comes to pain, there is little information about studies assessing CBD alone for pain treatment and their methodologies are weak [7]. Also, the studies on other kinds of cannabinoids products showed that the effectiveness differs in the type of pain [14].
The general situation in CBD use for treatment of anxiety, schizophrenia and pain is that, there are many studies existing, and some of them showed significant mitigating effects. But unfortunately, these studies have very small sample sizes and the data of chronic impact of the CBD product is missed. The reason explaining current situation may be considered as the complex process needed for conducting studies involving cannabis-related substances. The following information is quoted from FDA website (https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd)
Much more data on long-term safety and effectiveness is needed to prove using CBD is an effective strategy [7].
Conducting clinical research using cannabis-related substances that are scheduled by the DEA often involves interactions with several federal agencies. This includes: a registration administered by the DEA; obtaining the cannabis for research from NIDA, within the National Institutes of Health, or another DEA-registered source; and review by the FDA of the IND or INAD application and research protocol.
Conclusion
The difference between high-dose CBD and low-dose CBD use is so large, making it hard to talk about risk in low-dose CBD use using data and conclusion from clinical trial
Currently only one drug product is approved by FDA
Quality control of non-FDA-approved forms of CBD: investigation showed that only 45% of CBD oils was accurate labeled
User-experience-based effect of low-dose CBD use:
Most participant reports using CBD with a dosage lower than 50 mg per day
The main reason for use are self-perceived anxiety and sleep issue.
Generally, the surveyed CBD users reported that CBD helps in their problems, especially for those use for self-perceived anxiety.
high-dose CBD use based on clinical trials:
An effective new option for refractory seizures in Dravet syndrome and Lennox-Gastaut syndrome
Promising for premedicating before anxiety-inducing events such as public speaking and the chronic treatment of patients with schizophrenia.
Data for treating pain is limited and weak, and the effectiveness may differ in the type of the pain
Not risk free, has both drug interaction and adverse event potential
Generally sample sizes are small and the data of chronic impact of the CBD product is missed. Longer-term safety and effectiveness data are critically needed to check balance of benefit to harm
References
[1] J. Moltke and C. Hindocha, “Reasons for cannabidiol use: a cross-sectional study of CBD users, focusing on self-perceived stress, anxiety, and sleep problems,” J. Cannabis Res., vol. 3, no. 1, 2021, doi: 10.1186/s42238-021-00061-5.
[2] N. Masataka, “Anxiolytic Effects of Repeated Cannabidiol Treatment in Teenagers With Social Anxiety Disorders,” Front. Psychol., vol. 10, no. November, 2019, doi: 10.3389/fpsyg.2019.02466.
[3] M. M. Bergamaschi et al., “Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-nave social phobia patients,” Neuropsychopharmacology, vol. 36, no. 6, pp. 1219–1226, 2011, doi: 10.1038/npp.2011.6.
[4] J. A. S. Crippa et al., “Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report,” J. Psychopharmacol., vol. 25, no. 1, pp. 121–130, 2011, doi: 10.1177/0269881110379283.
[5] A. W. Zuardi et al., “Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life,” Front. Pharmacol., vol. 8, no. MAY, pp. 1–9, 2017, doi: 10.3389/fphar.2017.00259.
[6] P. T. Kocis and K. E. Vrana, “Delta-9-Tetrahydrocannabinol and Cannabidiol Drug-Drug Interactions,” Med. Cannabis Cannabinoids, vol. 3, no. 1, pp. 61–73, 2020, doi: 10.1159/000507998.
[7] C. M. White, “A Review of Human Studies Assessing Cannabidiol’s (CBD) Therapeutic Actions and Potential,” J. Clin. Pharmacol., vol. 59, no. 7, pp. 923–934, 2019, doi: 10.1002/jcph.1387.
[8] M. O. Bonn-Miller, M. J. E. Loflin, B. F. Thomas, J. P. Marcu, T. Hyke, and R. Vandrey, “Labeling accuracy of cannabidiol extracts sold online,” JAMA - J. Am. Med. Assoc., vol. 318, no. 17, pp. 1708–1709, 2017, doi: 10.1001/jama.2017.11909.
[9] A. Hazekamp, “The Trouble with CBD Oil,” Med. Cannabis Cannabinoids, vol. 1, no. 1, pp. 65–72, 2018, doi: 10.1159/000489287.
[10] R. Talati et al., “Effectiveness and Safety of Antiepileptic Medications in Patients With Epilepsy.” Agency for Healthcare Research and Quality (US), Rockville (MD), 2011, [Online]. Available: http://europepmc.org/books/NBK83937.
[11] P. McGuire et al., “Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial,” Am. J. Psychiatry, vol. 175, no. 3, pp. 225–231, 2018, doi: 10.1176/appi.ajp.2017.17030325.
[12] D. L. Boggs et al., “The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial,” Psychopharmacology (Berl)., vol. 235, no. 7, pp. 1923–1932, 2018, doi: 10.1007/s00213-018-4885-9.
[13] F. M. Leweke et al., “Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia,” Transl. Psychiatry, vol. 2, no. January, 2012, doi: 10.1038/tp.2012.15.
[14] M. R. Amin and D. W. Ali, “Pharmacology of Medical Cannabis,” Adv. Exp. Med. Biol., vol. 1162, pp. 151–165, 2019, doi: 10.1007/978-3-030-21737-2_8.
この記事が気に入ったらサポートをしてみませんか?
