
I-3: Disclosure of Payments to HCPs
This article summarizes the contents of 3️⃣ Disclosure of payments to HCPs, "Chapter 1: Basic Structures of Regulations on Transfer of Value and Bribery to Healthcare Professionals".
For the Japanese version, please refer to the following
See below for related articles.
(1)Regulations by law
1)Clinical Trials Act
In response to the so-called Diovan scandal, in order to restore trust and ensure transparency in Japanese clinical research, the Review Committee on the System for Clinical Research concluded that legal regulations are necessary for the provision of funds for clinical research from pharmaceutical companies to HCPs. Accordingly, the Clinical Trials Act has been effective since April 1, 2018.
The purpose of the Clinical Trials Act is to “promote the conduct of clinical trials through ensuring the confidence in clinical trials of citizens including clinical trial subjects in order to contribute to the improvement of public health and hygiene” (Article 1). And the Act obliges pharmaceutical companies to conclude contracts to provide funding for the specified clinical trials (Article 32) and to publish the funding for such trials (Article 33).
2)Subject and method of disclosure
Pharmaceutical companies shall publicize research funds, donations, manuscripts, or lecture fees, etc. The recipients subject to the disclosure include the person responsible for the clinical research, the institution to which the person responsible belongs, and the organization who controls and manages the research funds.
Obligation for disclosure started from the business year beginning on or after October 1, 2019, and the disclosure shall be made within 1 year after the end of each business year. The duration of the disclosure is 5 years from the disclosure.

(2) Self-regulations
1) JPMAʼs Transparency Guideline
The World Medical Association (“WMA”) states in the WMA Statement concerning the Relationship between Physicians and Commercial Enterprises that although “industry support enables the furtherance of medical research, scientific conferences and continuing medical education that can be of benefit to patients and the entire health care system”, “conflicts of interest between commercial enterprises and physicians occur that can affect the care of patients and the reputation of the medical profession” so therefore, “rather than forbidding any relationships between physicians and industry, it is preferable to establish guidelines for such relationships. These guidelines must incorporate the key principles of disclosure, avoidance of obvious conflicts of interest and the physicianʼs clinical autonomy to act in the best interests of patients”.
In addition, the Final Recommendation issued on April 28, 2010 by the Review Committee for Pharmaceutical Administration for the Verification and Prevention of Recurrence of Hepatitis Drug Injuries required appropriate management of conflict of interest and measures to enhance transparency that had been conducted overseas be taken.
Considering these backgrounds, JPMA issued the “Transparency Guideline for Relation between Corporate Activities and Medical Institutions” (the “Transparency Guideline”) in 2011. The Transparency Guideline was last revised in September 2018 in response to the enactment of the Clinical Trials Act.
The purpose of the Transparency Guideline is to gain a wide understanding of the pharmaceutical industryʼs contribution to life sciences, such as medicine and pharmacy, and for corporate activities to be conducted with high ethical standards, by making the relation between member companiesʼ activities and HCPs/HCOs transparent. Each member company is expected to prepare its own in-house policy for transparency as a code of practice, referring to the Transparency Guideline.
2)Recipients subject to disclosure and method thereof
The recipients subject to the disclosure are as follows.
(ⅰ)Medical Institutions;
(ⅱ)Research Institutions;
(ⅲ)Healthcare-related organizations;
(ⅳ)Foundations;
(ⅴ)Healthcare professionals;
(ⅵ)Researchers in medicine, pharmacy, or life sciences of science or engineering, etc.
In addition, the following items are subject to disclosure.
(1)Research and development expenses
Disclosure shall be made in accordance with the following procedures, together with the annual total amount of expenses incurred in R&D and post-marketing drug surveys for prescription drugs.

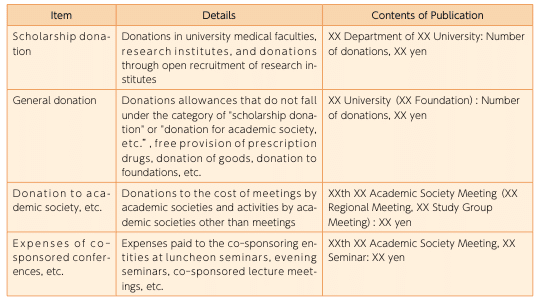
(2)Academic research support expenses
Funds provided for promotion of academic research will be publicized in the following manner, together with the total annual amount for each item.
(3) Manuscript/writing fees, etc.
For provision of scientific information on in-house drugs, medicine, and pharmacy, as well as for fee as consideration for lectures and writing or supervision of the manuscript that are related to research and development, or commissioning services including consulting contracts, etc. will be publicized in the following manner, together with the total annual amount for each item.

(4) Information provision related expenses
Expenses of lecture meetings and explanation meetings, for providing information, etc. related to in-house products, medicine and pharmacy to medical professionals will be publicized in the following manner.

(5)Other expenses
Expenses for hospitality, etc. as social courtesy will be publicized in the following manner.

(3)Sanctions against violation
Under the Clinical Trials Act, MHLW may recommend pharmaceutical companies publicize the relevant information, the Minister may publicize the name of the company when and the company does not comply with the recommendation.
In addition, MHLW may, to the extent necessary, request that a person who conducts specified clinical trials submit the necessary report or books, documents or other items, or have the Ministryʼs official enter the workplace of such person to inspect books, documents or other items or to question persons concerned (Article 35 of the Clinical Trials Act). If a pharmaceutical company refuses to submit a report or makes a false report, a fine of not more than ¥300,000 will be imposed (Article 42 of the Clinical Trials Act).
For the Japanese version, please refer to the following
See below for related articles.
The full text and attachments are available at the following links (Japanese only).
この記事が気に入ったらサポートをしてみませんか?
