Mistakes in Quantum Mechanics as Seen from the History of Science (5)
The orbital electrons are loosely tethered to the proton and electron charges of the nucleus and fall into the gamma-ray standing wave node generated by the neutrino plunge into the nucleus. It turns out that the electron is not a stochastic entity due to the wave function. Einstein was correct in his assertion.
Quantum mechanics predicts physical properties fairly accurately. Prediction of phenomena by calculation is the basis for thinking that quantum mechanics is correct. However, quantum mechanics cannot explain nuclear transmutation such as cold fusion because the internal structure of atoms is unknown. On the other hand, the physical properties of semiconductors can be described quite accurately. The reason is that when I introduced the wave function, I used statistical mechanics.
The introductory part of learning quantum mechanics is very confusing. The introduction of the hydrogen atom and the use of double slits to explain the wavefunction are far from reality.
Non-existent hydrogen atoms, non-interfering slits
When studying quantum mechanics, the hydrogen atom is used as an example of a wave function. However, in nature there is no such thing as one proton and one electron. A hydrogen molecule has two protons bound by two electrons.

In space, interstellar matter exists, but much of it is in the form of protonated hydrogen molecules, which are three protons bound by two electrons. Both are mechanically stable structures.

When low-pressure hydrogen gas is put into a glass chamber and a high voltage is applied, the hydrogen molecules enter a plasma state in which protons and electrons are separated. The emission line spectrum of hydrogen atoms discovered by Ballmer was looking at plasma emission.
Also, the double-slit experiment, which is often used in quantum mechanics, is an experiment that uses a large number of electrons.

An implicit assumption in the double-slit experiment is that electrons are randomly shot into the slit. Electrons that hit the slit electrify the slit, but electrons that pass near the slit are bent by the electrification of the slit. These are usually not explained at all. Why was such a strange experiment carried out?
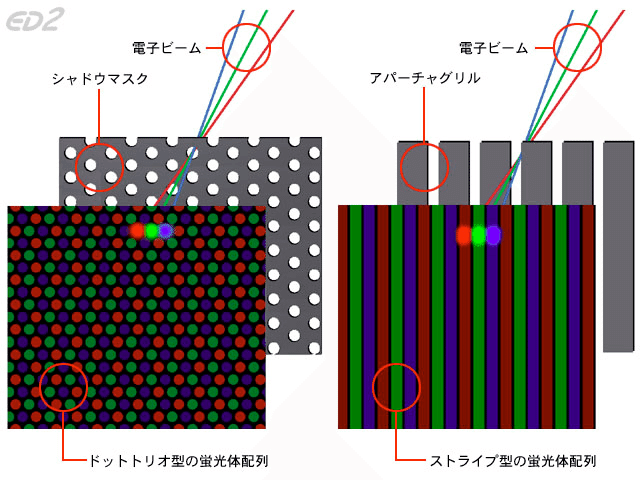
The interference fringes of the double slit had a hidden element to make them interfere. In fact, the cathode-ray tubes used in color televisions used to control electron beams with such precision that no interference appeared even through slits.
Quantum mechanics is statistical mechanics
Schrödinger said, "Quantum mechanics is born from statistical mechanics and ends in statistical mechanics."
Statistical mechanics is ``the field of physics that applies the laws of mechanics, electromagnetic law, and probability theory to the motion of the many particles that make up matter, and discusses the macroscopic properties of matter using the law of statistical averages.''
The technology of the early 20th century could not handle a single atom. Even now, except for special cases, humans do not have the technology to handle a single particle. The characteristics of quantum mechanics are obtained from experiments using a large amount of electrons and atoms.
Planck discovered discrete energy from the temperature of the blast furnace. The concept of quantum has been statistical from the beginning. Bohr's atomic model was inspired by the spectrum of the hydrogen atom measured by Balmer. This is also the result of discharging in a chamber containing low-pressure hydrogen gas. A large amount of hydrogen plasma is emitting light. De Broglie waves were invented to explain a statistical phenomenon. The wave function represents the probability distribution of electrons.
The experiment of Debison and Germer, which verified the de Broglie wave, was also done with a large amount of electrons and nickel. This is also a phenomenon of statistical mechanics.

The nineteenth century was the age of thermodynamics. Most of the researchers who established quantum mechanics were experts in thermodynamics and well-versed in statistical mechanics. Einstein also spent much of his research life in thermodynamics.
As a result of quantum mechanics trying to treat a single electron statistically, the logic got twisted somewhere. I tried to theorize it with the uncertainty principle, but it gave me a rather incomprehensible result. It can be said that Einstein's intuition was correct when he refuted quantum mechanics from start to finish.
And it was all young researchers who claimed the theory underlying quantum mechanics.
• Einstein 1879-1955 Age 26
• Heisenberg 1901-1976 Age 26
• Schrödinger 1887-1961 Age 39
• Bohr 1885-1962 Age 28
• de Broglie 1892-1987 Age 32
• Hideki Yukawa 1907-1981 Age 27 When he published his theory that age is important
Due to the effects of World War I and the Spanish Flu, mid-career researchers disappeared, and young researchers emerged. Quantum mechanics is the result of youthful determination to make some groundbreaking discoveries.
It is interesting to note that the slightly older Einstein and Schrödinger were against quantum mechanics. The rise of quantum mechanics did not come from old researchers thinking about classical physics who understood new theories and changed their minds. Few researchers switched from classical physics to quantum mechanics. Even Heisenberg, creator of the uncertainty principle, recalled that it was very difficult to let go of the old notions of classical physics. The reason why quantum mechanics spread is that old researchers left the research stage.
Quantum mechanics may survive as statistical mechanics. However, a theory that excludes quantum mechanics should be needed for the essential structure of atoms and nuclei.
Advocating for SEAM
A neutron is a composite particle that has an electron bonded to a proton. The nucleus is bound together by protons and electrons in excited states of the protons. The orbital electrons are loosely tethered to the plus and minus of the nucleus and fall into the nodes of the standing wave of gamma rays emitted from the nucleus. I would like to call this the Static Electron Atom Model-SEAM.
Cold fusion cannot be explained by current quantum mechanics, so LENRs (Low Energy Nuclear Reactions) are often underappreciated. However, if LENR is ignored, physics will stagnate and energy development will not proceed to the next step. The LENR has already advanced enough to change the standard theory. It will be necessary to advance the theory according to the discovered phenomena.
From the next time, I would like to change the title and explain the structure of atomic nuclei, magnetism, heat, etc. by SEAM.
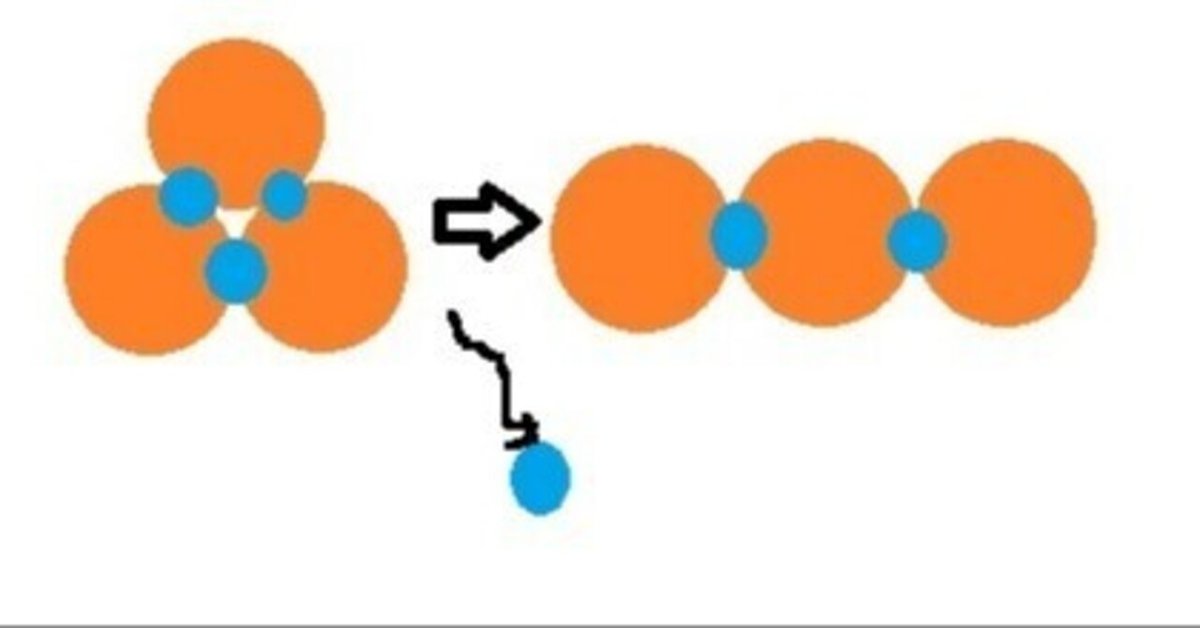
The title image is a diagram of tritium releasing one electron and becoming helium 3.
この記事が気に入ったらサポートをしてみませんか?
