
Mistakes in Quantum Mechanics as Seen from the History of Science (1)
prelude to thinking
Before we start, I want to write a little long introduction. We learn many things in school, but there is one thing we do not learn. It's the right way to think. At school, we test how accurately we memorize textbooks and what teachers say. However, if you think about it on your own, you will be severely criticized. Not only knowledge but also thoughts are taught as knowledge, but this is the same as being disciplined not to think. Thinking is the same as sports, and you can't do it unless you practice. You need the right age and the right training. Most people are forced into 12 to 16 years of unthinkable training in school. It goes without saying that the more educated you are, the less you will be able to think rationally.
What is "scientific" thinking? Science often says that we can think on the shoulders of giants. But what if no one got the giant's shoulder wrong? I don't think so. In fact, Descartes gave the correct answer in the 17th century as to how to think scientifically. Descartes advocated a mechanical worldview, saying that nature is made up of a combination of things like a mechanical clock. The mechanical worldview is the basis for the important ideas of science: proximity action and reductionism. The Scientific Revolution of the 17th century succeeded in soliciting donations from aristocrats and merchants by advocating practical science. It was the mechanical worldview that underpinned practical science.
If natural phenomena are caused by combinations of things, then nature can be manipulated by manipulating things. In the 17th century, nature threatened humans with overwhelming threats. Humans could only handle horses to carry a small amount of cargo, and use the force of the wind to propel them weakly across the sea. The mechanical world view showed the possibility that humans could compete against the mighty nature. The industrial revolution that gave birth to the modern era is based on a mechanical worldview.
It's a proximity action where things come in contact with each other and transmit power. However, Newton's universal gravitation, which appeared in the 17th century, was a distant action. Moreover, since gravitation alone cannot maintain the planet's orbit, Newton, who was a devout Christian, believed that the power of God was at work at the heart of the orbit. He hid God at the center of science. "God is dead," said Nietzsche in the 20th century, but Nietzsche didn't realize this either.
Descartes argued that the reason why planets in erratic orbits do not collide is that some kind of repulsive force is generated from the planets, and advocated the vortex theory.

However, Newton's universal gravitation resulted in ignoring this repulsive force. This continues today.
He also believed that it was the liquid-like filling of the universe—the ether—that conveyed the forces of attraction and repulsion of the planets. It's a proximity action, not a distance action. Ether has changed its shape and remains as "field" and "space".
After the voltaic battery was invented at the end of the 18th century, electricity became the subject of research in the 19th century. Many researchers, including Faraday, experimented with electricity. Faraday thought that when magnets were brought closer together, they would repel or attract each other. Even before Faraday, there was the concept of power to explain proximity action. Faraday considered the force to be the cause of the electromagnetic force, and took it as a concept close to the current field.
However, the place is not a thing. It is a concept created by humans to better understand natural phenomena. From this point on, it seems that electromagnetism came to include errors. It is believed that the electric lines of force that represent the transmission of the Coulomb force are neutralized in plus and minus.
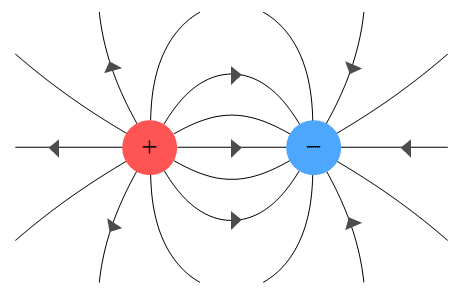
However, the Coulomb force itself does not have the ability to sense the opponent. It just goes straight.
The reason why the Coulomb force seems to neutralize in the middle is that we are seeing how the vector of the force acting on the object is synthesized inside. This will be an important element in the main story.

Michelson-Morley's experiments at the end of the 19th century denied the aether, but left the field of electromagnetism intact.
There is another important way of thinking. It is often said that ``the basic idea of natural science is that the laws that govern natural phenomena exist behind them,'' but this is an outrageous misunderstanding. If a seemingly simple natural phenomenon is caused by a complicated mechanism, this way of thinking cannot be used to investigate the cause. As will be explained in another article, gravity seems simple but has a complex mechanism.
The idea that "the laws behind natural phenomena are represented by mathematical formulas" is based on the religious idea of Pythagoreanism. In the theory of relativity, natural phenomena are often explained by transforming mathematical formulas. Of course, it is incompatible with the mechanical worldview.
Where did quantum mechanics go wrong
Sorry for the long introduction, but let me explain why quantum mechanics is wrong. Before that, let's look back at the history of the establishment of quantum mechanics.
• 1895 Perrin discovers the negative charge of cathode rays
• 1899 Thomson, calculating the charge of an electron
• Separation of Rutherford, alpha and beta rays
• 1900 Discovery of the Planck distribution → It was found that the formula expressing the energy of the cavity radiation shows discrete values. Quantum Hypothesis of Energy
• 1905 Einstein's "Light Quantum Hypothesis" "Theory of Brownian Motion"
"Special theory of relativity" ← Planck accepted
• 1911 Millikan successfully separates electrons Rutherford presents atomic model
• 1913 Bohr's atomic model 1914-1918 World War I
• 1918 Rutherford discovers the proton 1918-1921 Spanish Flu
• 1920 Rutherford's nuclear electron theory
• 1923 Compton effect confirms light quantum hypothesis
• 1924 introduction of de Broglie waves
• 1925 Heisenberg Determinant. Pauli exclusion principle
• 1926 Schrödinger equation
• Einstein God does not play dice
• 1927 Heisenberg, Uncertainty Principle Copenhagen Interpretation.
• 1931 Chadwick, discovery of the neutron
• 1932 von Neumann denying hidden variables
• 1933 Hideki Yukawa, Doubts on Neutrons
• 1935 Schrödinger's Cat EPR Paradox
• 1939 World War II
As a historical image, quantum mechanics seems to have developed in a straight line from the beginning of the 20th century. However, quantum mechanics was established after the discovery of the neutron in 1931. It became more popular after World War II from 1955 to the 1970s.
Many discussions were held in the process of establishing quantum mechanics, but I tried to summarize the main points.
• Avoid Planck quantum theory and minimize its influence
• Rutherford Atomic nuclei are composed of protons connected by negative electrons - intranuclear electron theory
• Schrödinger Schrödinger's Cat Wave packet convergence
• Einstein God does not roll dice Hidden variables?
• Hideki Yukawa Neutrons emit electrons and become protons. Aren't mesons like electrons? Neutrons as composite particles
Planck, who discovered the quantum, was a lifelong skeptic of quantum mechanics. Schrödinger also points out that this is because quantum mechanics was born from statistical mechanics.
Also, although most people may not have heard of it, Rutherford's nuclear electron theory dominated in the 1920s. Rutherford argued that electrons exist inside the nucleus from experiments on beta decay. Chadwick's discovery of the neutron disproved this, and quantum mechanics became the mainstream of physics. At that time, Hideki Yukawa made a note of his doubt that neutrons are composite particles in which protons and electrons are combined.
Quantum mechanics was born to explain Bohr's atomic model. From Rutherford's atomic model and Balmer's spectral lines, Bohr assumed that orbital electrons have discrete radii.
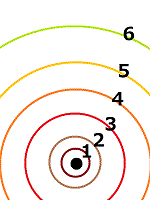
To explain this, de Broglie of France argued that electrons are in a wave-like state, de Broglie waves. De Broglie waves are the origin of quantum mechanics. Not Planck's quantum. At present, the probabilistic existence of electrons, which is often said, comes from de Broglie waves.
It was Heisenberg who expressed the periodic motion of an orbital electron, which is a de Broglie wave, in a determinant. Soon after Schrödinger expressed the same thing as a linear equation, both were mathematically the same. As an aside, the basis of quantum computers is that the Heisenberg determinant and the Schrödinger equation are mathematically the same.
Note that orbital electrons have jumpy radii—quantum jumps are expressed in the Schrödinger equation, but the cause is never mentioned. The mechanism of the Pauli exclusion principle, which states that electrons with the same spin cannot enter the same orbit, remains unclear. These two only explain phenomena with mathematical formulas, and do not explain the relationship between things. It is Pythagoreanism*, not a mechanical worldview.
*Religious doctrine that nature should be expressed in mathematical formulas
Rutherford's Nuclear Electron Theory Survives
Let's look again at the problems from the end of the 19th century to around 1934.
Why don't orbital electrons fall in Bohr's atomic model?
Also, why do electron orbits take discrete values? quantum leapDe Broglie waves were introduced to satisfy these two requirements. The background to this era was thermodynamics.
• de Broglie waves are derived from statistical mechanics → probability distribution of electrons
• Most of the physicists who built quantum mechanics were experts in thermodynamics
• The 19th century was the age of thermodynamics!
• Boltzmann → Planck, Maxwell also thermodynamics
• Schrödinger → Quantum mechanics is born from statistical mechanics and ends in statistical mechanics
• Einstein → hidden variable
Quantum mechanics can be said to have been born out of trying to capture a single electron statistically. I wrote that Rutherford's nuclear electron theory was the mainstream for about ten years until the discovery of the neutron. The nuclear electron theory was scrapped due to several counterarguments, but if we look at the specific reasons, we can see that it is not sufficient. The English version of wikipedia has a content that denies the nuclear electron theory in the neutron section.
Rutherford's nuclear electron theory was refuted, but it was not suitable.
So far, I have explained that the introduction of the wave function was not appropriate, and that the nuclear electron theory has survived. So what should we do? "Bohr's atomic model was not wrong." Next time, I will think about the nuclear electron theory "correctly".
この記事が気に入ったらサポートをしてみませんか?
