Mistakes in Quantum Mechanics as Seen from the History of Science (2)
Last time, I pointed out that there is a mistake in quantum mechanics in the 1924 de Broglie wave. Rutherford's theory of electrons in the nucleus of 1920 is still valid. Why was the de Broglie wave, which makes electrons stochastic, introduced? "Let's look back from the atomic model."
before de Broglie wave
The idea that when we divide matter into smaller pieces, we end up with atoms, dates back to ancient Greece, but it is relatively new that we started thinking about the internal structure of atoms. Thomson, who discovered electrons in 1904, proposed a model that electrons exist in atoms like grape bread.

Thomson's atomic model did not yet have a nucleus. Rutherford discovered the atomic nucleus in 1911. When positively charged alpha rays emitted from radium are shot into gold foil, they may bend or bounce straight back. We found that there are clumps with positive charges. At this time protons were still unknown. He also discovered protons at Rutherford in 1918.
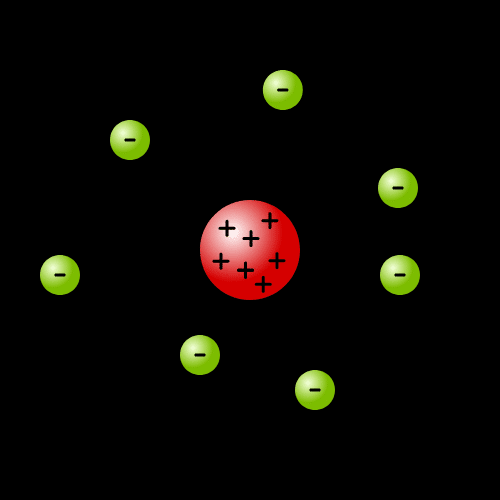
In 1913, Bohr added Balmer's spectrum to Rutherford's atomic model, and proposed Bohr's atomic model in which the surrounding electrons have periodic orbits.

In Bohr's atomic model, negative electrons were thought to revolve around a positively charged nucleus. Since electrons and nuclei attract each other, centrifugal force should keep them in the electronic state. However, according to the interpretation of classical mechanics at the time, it was predicted that rotating electrons would receive acceleration, emit electromagnetic waves, lose momentum, and fall into the nucleus. But atoms exist. Orbital electrons do not fall into the nucleus. The search for this mechanism led to quantum mechanics.
Rutherford discovered the proton in 1918. Based on this, in 1920, he announced the intranuclear electron theory, in which electrons exist inside the nucleus. The nucleus was thought to be made up of protons and electrons. This paper can be read online.
In 1920, at this time, the nuclear electron theory was accepted as the prevailing theory in the physics society. However, it seems that Rutherford began to leave the field of his experiments as his social status rose from around this time. The neutron was predicted by Rutherford, but Chadwick, who inherited his experiment, was given the credit for his discovery. Rutherford became president of the Royal Society in 1926.
In the chronology, de Broglie announced the de Broglie wave in 1924, but it was actually accepted after 1927. It was verified by an electron beam interference experiment. In addition, de Broglie waves were contrasted with Einstein's light quantum hypothesis, and it is said that if electromagnetic waves are particles, the particles may be waves.
No need for de Broglie wave
Now let's stop time in 1920. Although it was found that there is a negative charge inside the nucleus, it was still thought that the nucleus was affected only by the positive charge. This is Maxwell's understanding of electromagnetism that positive and negative electric lines of force neutralize along the way.

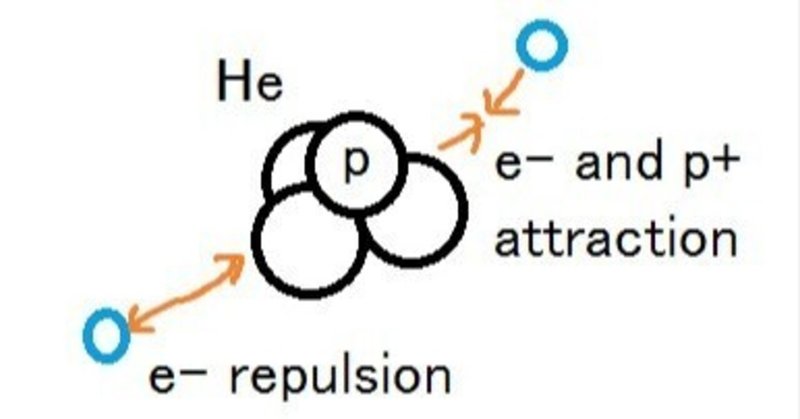
As explained last time, the electric lines of force do not neutralize on the way. Plus and minus reach the object separately, and the vector is combined inside the object. Applying this to the nuclear electron theory, the plus of protons inside the nucleus attracts electrons in orbit, but at the same time repels the electrons in the nucleus. Orbital electrons are states loosely bound to the plus and minus of the nucleus.
There is a video demonstrating this using magnets.
The magnet is attracted to N and S and repelled, and is loosely connected. The same thing happens inside the atom. In this case, there is no need to introduce the extraordinary phenomenon of de Broglie waves. Only a proper review of electromagnetism can explain it.

When pointing out that quantum mechanics is wrong, it is often the case that the uncertainty principle, the Copenhagen interpretation, etc. are discussed. However, there is little point in pointing out contradictions within quantum mechanics, and quantum mechanics will continue with minor modifications. Next time, let's explain the quantum jump.
この記事が気に入ったらサポートをしてみませんか?
